In this blog you will find class 6 to 12, IIT, NEET MATH & PHYSICS question and answer and also some articles about Science Engineering and Technology
1 Feb 2022
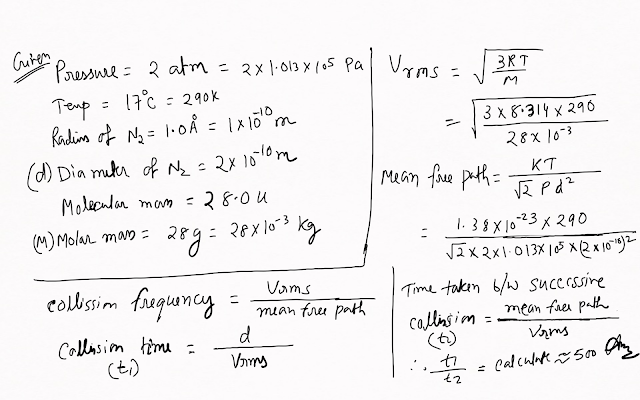
Estimate the mean free path and collision frequency of a nitrogen molecule in a cylinder containing nitrogen at 2.0 atm and temperature 17 °C. Take the radius of a nitrogen molecule to be roughly 1.0 Å. Compare the collision time with the time the molecule moves freely between two successive collisions (Molecular mass of N2 = 28.0 u).
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment
Please do not enter any spam link